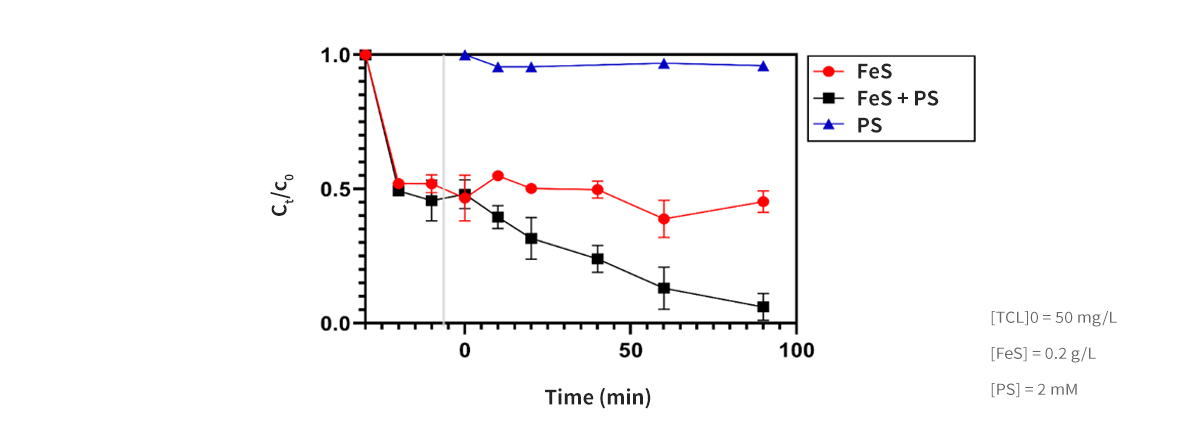
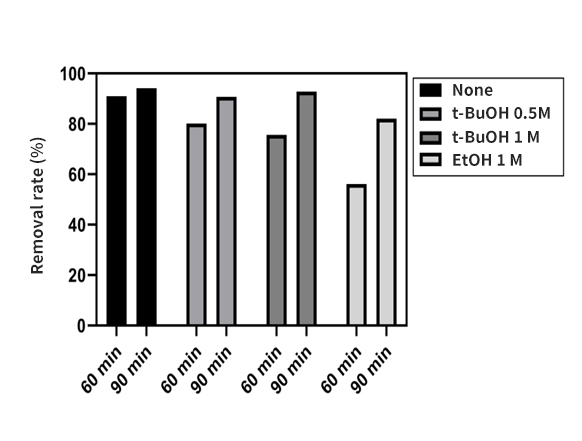
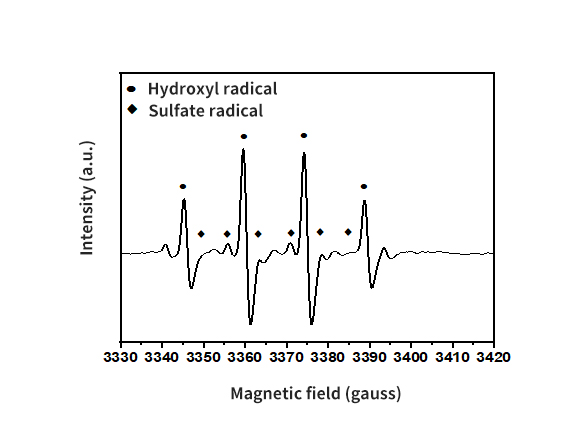
Development of materials and processes derived from recycled waste
Development of a process to build a resource circulation society by recycling waste desulfurization agent discharged from desulfurization facilities and using it as a Fenton oxidation catalyst for the treatment of hard-to-decompose wastewater.
Introduce
Development of materials and processes derived from recycled waste
Fenton oxidation catalyst overview
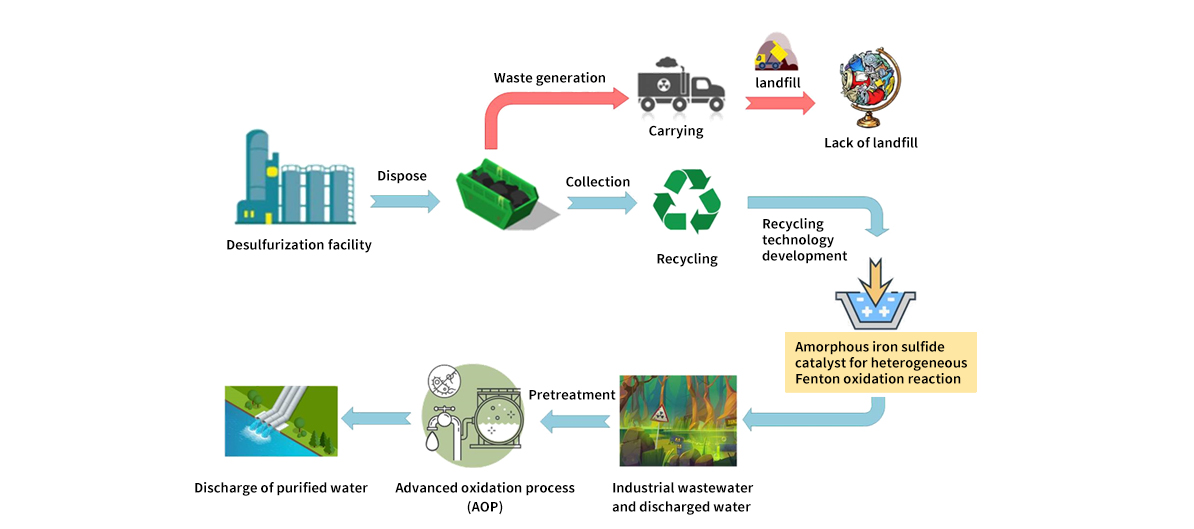
Characteristics of Fenton Oxidation Catalyst
Catalyst production through post-treatment and molding of spent desulfurization agent
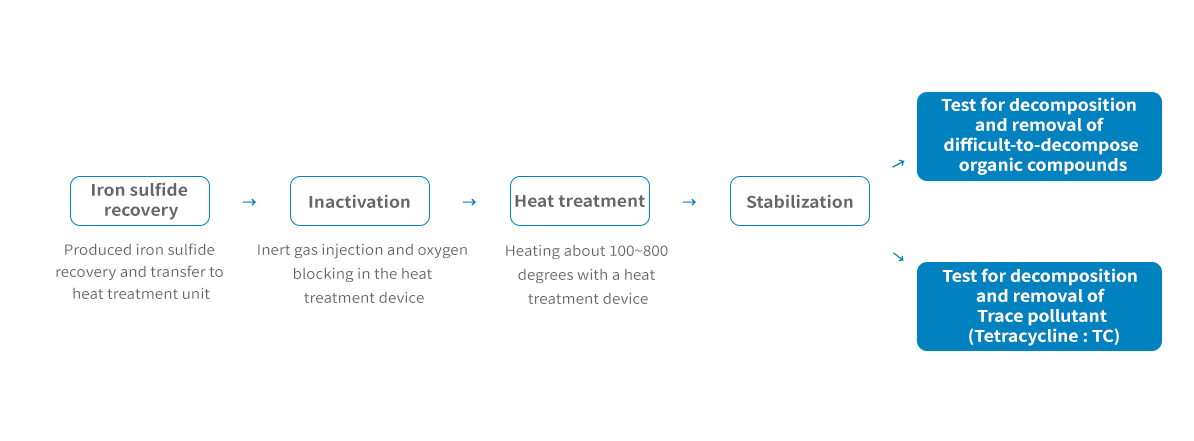
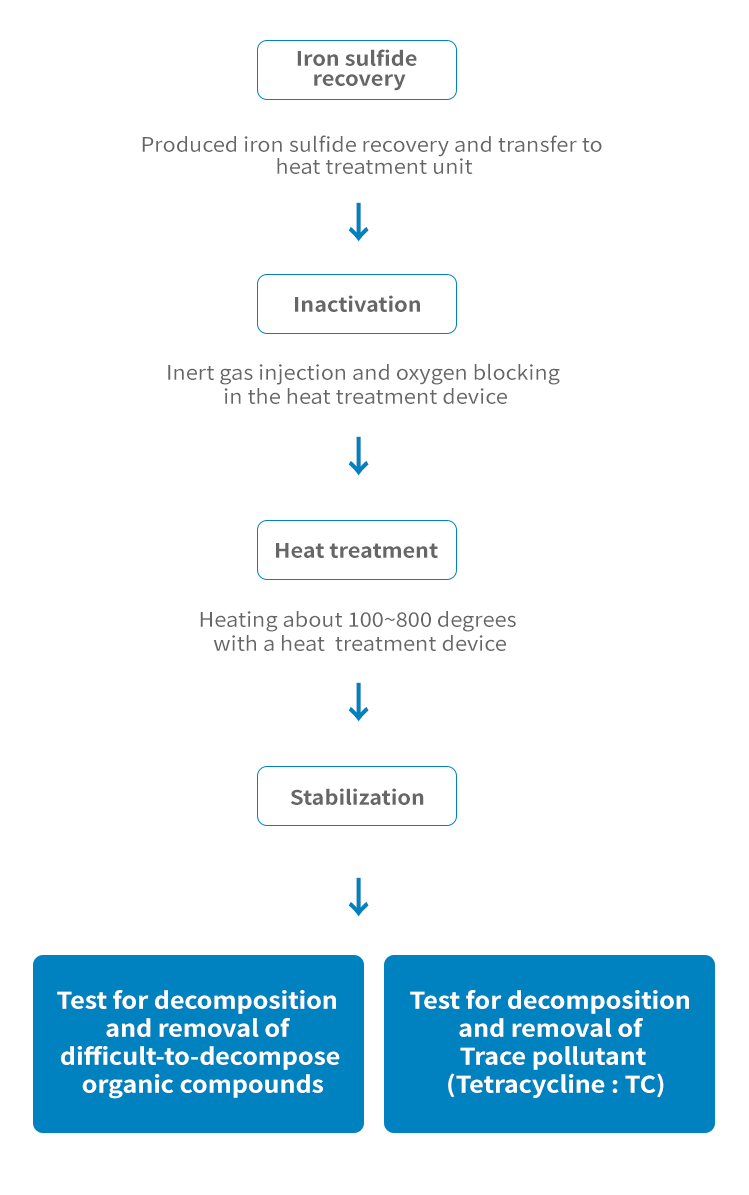
Catalyst Manufacturing method